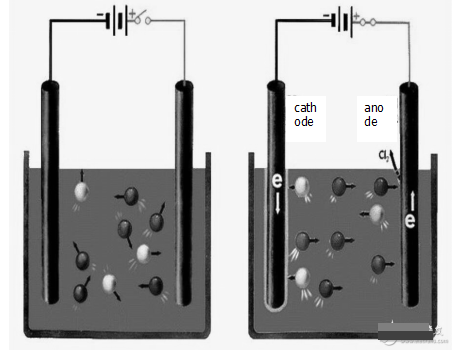
What is the difference between a primary battery and an electrolytic cell?
Electrolysis cell overview
The main application of the electrolytic cell is for the industrial high purity metal, which is a device for converting electrical energy into chemical energy (constitution: external power supply, electrolyte solution, yin and yang electrode). The process of reducing the oxidation reaction is caused at the anode and cathode by passing an electric current through the electrolyte solution or the molten electrolyte. The process of causing a current to pass through an electrolyte solution or a molten electrolyte to cause a reductive oxidation reaction on the anodes and cathodes is called electrolysis. A device that converts electrical energy into chemical energy is called an electrolytic cell or an electrolytic cell. When ions reach the electrode, electrons are lost or acquired, and the process of redox reaction is called electrolysis, which consumes electricity.
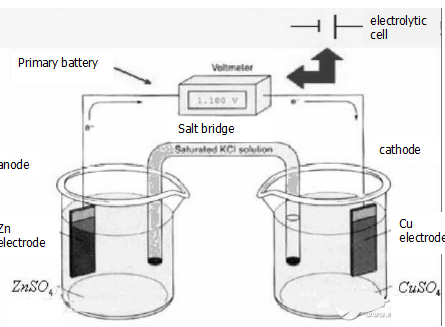
Primary battery overview
The history of the invention of the galvanic cells dates back to the end of the 18th century, when the Italian biologist Galvani was conducting a famous frog experiment. When a metal scalpel was used to contact the frog legs, the frog legs were found to twitch. The famous Volt believes that this is caused by the current stimulation between the metal and the frog leg tissue fluid (electrolyte solution). In 1800, Volt designed a device called a voltaic stack. Zinc is the negative electrode, silver is the positive electrode, and brine is used as the electrolyte solution. In 1836, Daniel invented the world's first practical battery and used it for early railway signal lights.
A device that generates a current by a redox reaction is called a primary battery, and can be said to be a device that converts chemical energy into electrical energy. Some primary batteries can constitute a reversible battery, and some primary batteries do not belong to a reversible battery. When the primary battery is discharged, the negative electrode undergoes an oxidation reaction, and the positive electrode undergoes a reduction reaction. For example, a copper-zinc primary battery, also known as a Daniel battery, has a positive electrode of a copper electrode and is immersed in a copper sulfate solution; the negative electrode is a zinc plate and is immersed in a zinc sulfate solution. The two electrolyte solutions are connected by a salt bridge, and the two poles are connected by wires to form a primary battery. The dry battery used in normal times is made according to the original battery principle.